The epiCMIT (epigenetically-determined Cumulative MIToses) mitotic clock (Durran-Ferrer, 2020) tracks DNA hypo- and hyper-methylation changes in heterochromatin and H3K27me3-containing chromatin, respectively, providing a comprehensive record of B cell mitotic history (pre- and post-malignant transformation). Higher epiCMIT levels correlate with poor survival in CLL and MCL, suggesting that a greater mitotic history predicts future proliferative capacity. However, no studies have investigated epiCMIT in splenic marginal zone lymphoma (SMZL).
We studied 142 SMZL cases, a rare lymphoid malignancy with specific genetic characteristics [del(7q), KLF2and NOTCH2 mutations, biased use of IGHV1-2*04]. After calculating epiCMIT scores from 450/850K Illumina arrays we compared these data with gene mutations from targeted resequencing and WGS, copy number variations, telomere length, RNA-Seq, miRNA-Seq data, and clinical outcome data.
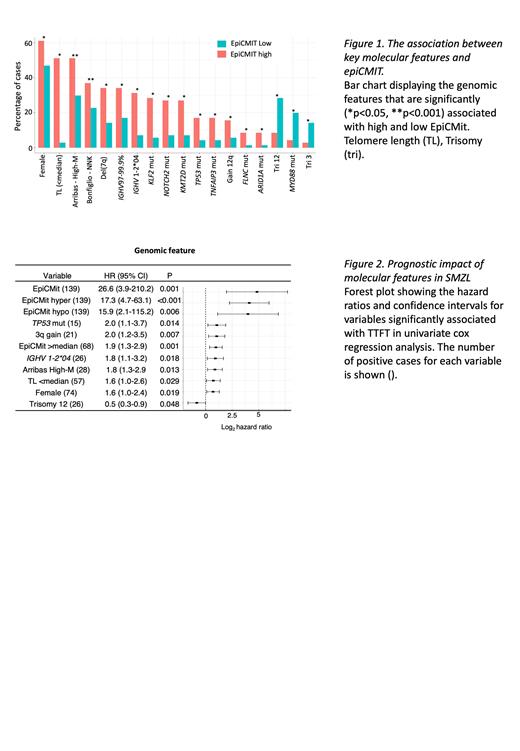
By defining epiCMIT-hyper [mean: 0.6, range: 0.29-0.91] and -hypo [mean: 0.71, range: 0.39-0.90] values in our cohort, we demonstrate a strong positive correlation between hyper- and hypo- scores, as previously observed in MCL and CLL (Durran-Ferrer, 2020). The highest score obtained for epiCMIT-hyper or -hypo per sample was then selected as the epiCMIT score per patient [mean: 0.72, range: 0.39-0.91]. We identified significant correlations between epiCMIT and key clinico-biological characteristics, including a significant negative correlation between epiCMIT and telomere length (R 2=0.67 p<0.001) supporting cell proliferation and concomitant telomere attrition. High epiCMIT scores were associated with female gender (p=0.02), IGHV1-2*04 alleles (p<0.001) with intermediate levels of IGHV somatic hypermutation (SHM) load (97-99.9%, p=0.04), driver somatic gene mutations, including those in KLF2 (p<0.001), NOTCH2 (p<0.01), TP53 (p=0.01) and KMT2D (p<0.001) and deletion of 7q (p=0.01) ( Fig1). Using previously defined classification systems (Arribas, 2015 and Bonfiglio, 2022), we show enrichment of high-risk subgroups [Arribas- high global methylation subgroup (High-M, p<0.001) and Bonfiglio- NFKB, NOTCH and KLF2 genetic subgroup (NNK, p=0.01)] in cases with high epiCMIT.
In a sub-cohort of 42 SMZL cases, with a spectrum of epiCMIT values and WGS/ (mi)RNA-Seq data available, we demonstrated 1) a significantly higher genome-wide somatic mutational burden in patients with high epiCMIT-total scores (25 v 17mut/Mb, p=0.001), and 2), correlations between epiCMIT and specific gene/miRNA expression associated with cellular survival and proliferation ( CARD11 [R 2=0.6, p<0.01], MAP2K1 [R 2=0.61, p<0.01], miRNA-155 [R 2=0.58, p=0.018]), apoptosis ( BCOR [R 2=0.61, p<0.01]), and epigenetic regulation ( EZH2 [R 2=0.58, p<0.01]).
We then conducted univariate Cox Proportional Hazards analysis to investigate the prognostic significance of clinical and molecular features on time to first treatment (TTFT; most prevalent treatments: splenectomy, n=51; rituximab, n=20) and overall survival (OS). The epiCMIT score and epiCMIT-hyper values showed the highest hazard ratios (HR) for TTFT (HR=26.6, p=0.001) and OS (HR=9.45, p=0.043) respectively ( Fig2). Other factors associated with shorter TTFT included TP53 mutation, 3q gain, IGHV1-2*04 usage, High-M, short telomeres and female gender. Shorter OS was significantly (p<0.05) linked to mutations in key genes (including TP53 and NOTCH2), genomic complexity and del(7q). To avoid redundancy and collinearity, only the most significant epiCMIT metric was included in a multivariate Cox Proportional Hazards model (backwards selection, 94 patients/63 events), and epiCMIT-total emerged as an independent marker for shorter TTFT (HR=44.4, p=0.004), together with gain of chromosome 3q (HR=2.6, p=0.002). Kaplan-Meier analysis further confirmed the importance of epiCMIT-total, with higher scores (>median: 0.73) associated with significantly shorter TTFT (median 23 vs 3.5 months) and higher mortality (p=0.004).
To conclude, we demonstrate the potential clinical utility of epiCMIT score in SMZL patients, identifying that SMZL patients with high epiCMIT harbour specific genetic characteristics, and exhibit reduced treatment-free survival. EpiCMIT could be valuable in clinical practice, helping to identify those patients destined to progress and require closer monitoring.
Disclosures
Scarfo:Janssen: Consultancy; BeiGene: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy; Lilly: Consultancy; Octapharma: Speakers Bureau. Ghia:Lilly/Loxo Oncology: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; MSD: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding. Rossi:AbbVie, AstraZeneca, Gilead, BeiGene, BMS, Janssen, Lilly, Kyte: Honoraria, Research Funding. Ahearne:Takeda: Other: Conference travel support; Pfizer: Research Funding. Forconi:Janssen-cilag: Honoraria, Other: Travel and accommodation, Speakers Bureau; beigene: Honoraria, Other: Travel and accommodation, Speakers Bureau; Astra-Zeneca: Honoraria, Speakers Bureau; Abbvie: Honoraria, Other: Travel and accommodation, Speakers Bureau. Thieblemont:Hospira: Research Funding; Gilead Sciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Janssen: Honoraria, Other: Travel Expenses; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Cellectis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Paris University, Assistance Publique, hopitaux de Paris (APHP): Current Employment; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kyte, Gilead, Novartis, BMS, Abbvie, F. Hoffmann-La Roche Ltd, Amgen: Honoraria; Bayer: Honoraria. Walewska:AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Meeting Attendance, Speakers Bureau; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Meeting Attendance, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees, Other: Meeting Attendance, Speakers Bureau; Secura Bio: Honoraria, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Meeting Attendance, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal